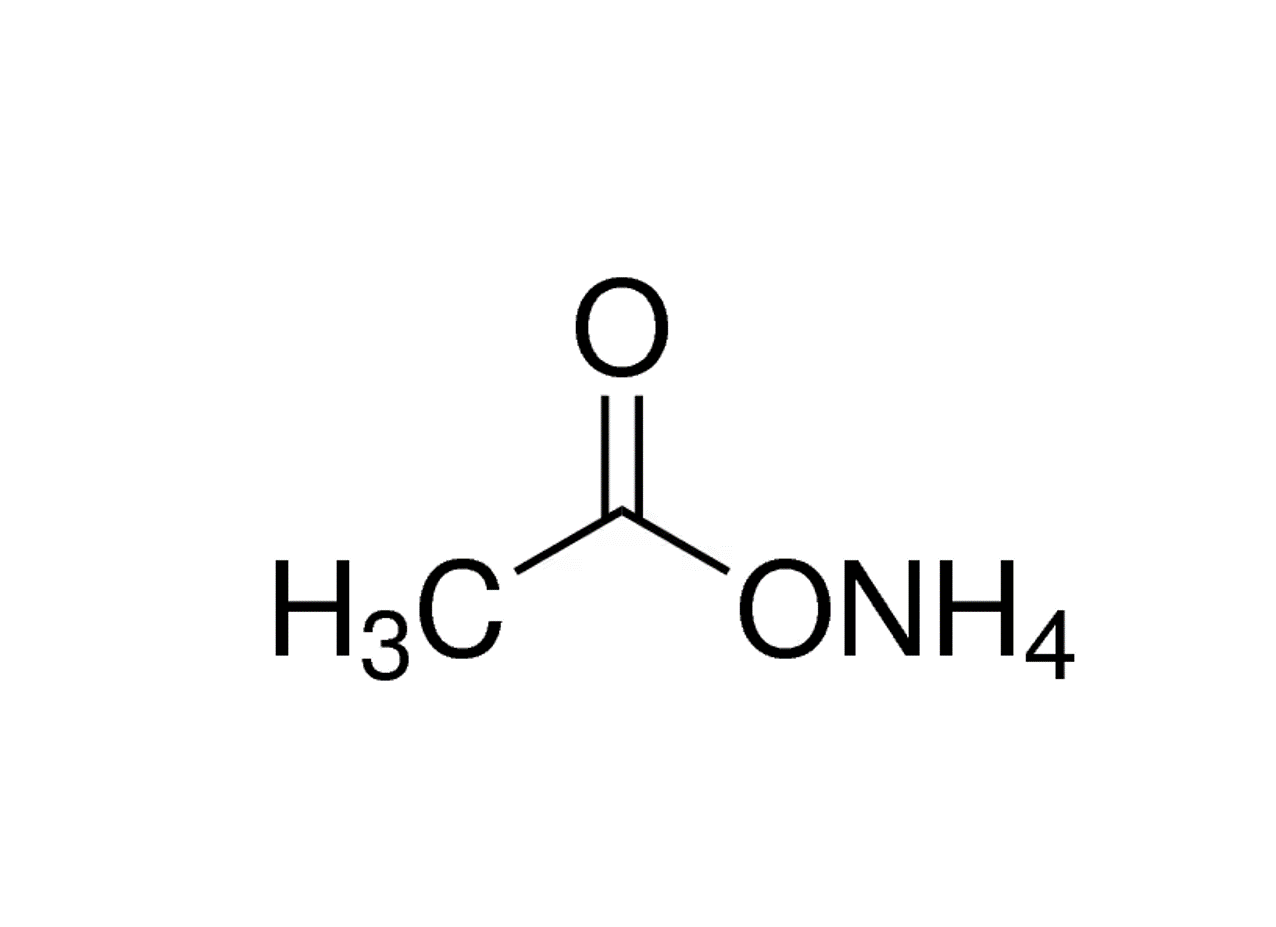
Sujata Nutri Pharma is one of the leading manufacturers and exporters of Ammonium Acetate CAS no.631-61-8
Ammonium Acetate – Properties: A buffer is a mixture of a weak acid and its conjugate base, or a weak base and its conjugate acid. The mixture resists changes in pH when small amounts of acid or base added. Buffers used in biochemistry to maintain the pH of a solution.
The buffer solution is made by mixing equal amounts of ammonia and acetic acid. The ammonia and acetic acid react to form ammonium acetate, which is a salt. The ammonium acetate buffer can resist changes in pH when small amounts of acid or base are added because it contains both an acid and a base.
Ammonium Acetate is used as a biodegradable de-icing agent.
Ammonium Acetate is used in the Knoevenagel condensation in organic synthesis.
Ammonium Acetate is used with distilled water to make a protein precipitating reagent.
In analytical chemistry, the compound is used in the form of a reagent. It is used as a reagent in different dialysis procedures for the elimination of contaminants through diffusion.
In agricultural chemistry, ammonium acetate, when used as a reagent, helps in determining soil CEC or cation exchange capacity along with the availability of potassium in the soil.